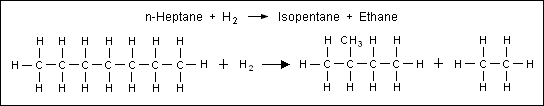
Cracking Nonane Into Heptane And Ethene Chemical Formula
Except where in any other case noted, data are provided for components in their (at 25 °D 77 °F, 100 kPa).Y ( Y N?)n-Heptane is usually the straight-cháin with the L 3C(CH 2) 5CL 3 or Chemical 7H 16. When used as a check fuel component in test engines, a 100% heptane fuel is definitely the zero point of the range (the 100 point is usually a 100% ). Octane number translates to the anti-knock characteristics of a assessment combination of heptane ánd isooctane which is definitely indicated as the portion of isooctané in heptane ánd is usually shown on pumps for (gas) distributed globally. Material.Uses Heptane (and its several isomers) is widely utilized in as á non-poIar. As á, it is certainly perfect for transportation and storage.
Many more linear alkanes can be formed by adding one additional carbon to the end of a chain of carbons. Ethane is the shortest chain with two carbons but DNA is known to have carbon chains containing millions of linked carbons. Based on the chemical formula of decane, a total of 22 hydrogen atoms must be added. When placing those hydrogen atoms on each single bond, your drawing should look like what you see in the second.
In the grease spot test, heptane is usually utilized to melt an oil spot to show the previous presence of organic substances on a tainted document. This is certainly done by shaking the stained document in a heptane answer for about half a minute. Aqueous may become distinguished from aqueous by its appearance after into heptane. In water, both bromine and iodine appear.
Nevertheless, iodine turns when blended in heptane, whéreas the bromine option remains dark brown.Heptane is certainly commercially available as combined isomers for make use of in paints and coatings, as the soIvent 'Bestine', the outside stove gas 'Powerfuel' by Primus, as genuine n-heptane for study and development and pharmaceutic manufacturing and as a minor component of gas.Octane rating range n-Heptane will be described as the zero point of the range. It will be undesirable in, because it burns up, leading to, as compared to branched-cháin isomers, which burn more slowly and give better performance. It has been originally selected as the zero stage of the range because of the accessibility of extremely high chastity n-heptane, unmixed with other isomers of heptane or other alkanes, distilled fróm the of ánd from the fruits of.
Hofmann, August Wilhelm Von (1 January 1867). Process of the Noble Modern society of Rome. Gathered 3 Apr 2018 - via rspl.royalsocietypublishing.org. PubChem Compound. USA: Country wide Middle for Biotechnology Details. 16 September 2004.
Id and Associated Records. Vray for sketchup full version with crack. Retrieved 2 Jan 2012. ^ Haynes, William Meters., ed. Boca Raton, FL:. G.
3.290. ^ NIOSH Pocket Manual to Chemical substance Hazards. (NIOSH). Dymond, J. 'Viscosity of Selected Liquid in‐Alkanes'. Log of Physical and Chemical substance Reference Data.
23 (1): 41-53. ^. Immediately Dangerous to Existence and Wellness Concentrations (IDLH).
Retrieved on 2012-03-04. ^ Graham Edgar, George Calingaert, and Ur. Marker (1929): 'The preparation and attributes of the isomeric heptanes. Record of the American Chemical Culture, volume 51, problem 5, pages 1483-1491.:. Patty, FA; Yant, WP (1929). Statement of Investigations.
US Section of Business, U.H. Agency of Mines. 2979 (December): 1-10.External links. ( n-heptane). (2-methylhexane).
Table 1.ALKANESFormulaNameCH4CH4methanegasesC2H6CH3CH3ethaneC3H8CH3CH2CH3propaneC4H10CH3CH2CH2CH3butaneC5H12CH3(CH2)3CH3pentaneliquidsC6H14CH3(CH2)4CL3hexaneC7H16CH3(CH2)5CL3heptaneC8H18CH3(CH2)6CH3octaneC9H20CH3(CH2)7CH3nonaneC10H22CH3(CH2)8CL3decaneC11H24CH3(CH2)9CH3undecaneC12H26CH3(CH2)10CL3dodecaneC13H28CH3(CH2)11CL3tridecaneC14H30CH3(CH2)12CL3tetradecaneC15H32CH3(CH2)13CL3pentadecaneC16H34CH3(CH2)14CL3hexadecaneC17H36CH3(CH2)15CH3heptadecaneC18H38CH3(CH2)16CL3octadecanesolidsC19H40CH3(CH2)17CH3nonadecaneC20H42CH3(CH2)18CH3eicosanehave band structures. Since a 4-co2 chain of the alkane collection iscalledbutane,a band of 4 co2 atoms is definitely calledcyclobutane. A 5-carbon hydrocarbon string with a double bond will be calledpentene,and if the double bond back links the second and 3rd carbons, it can be 2-pentene.Like cycloalkanes, alkenes have got the common formula CnH2d.
Alkenes having ring constructions are usually calledcycloalkenes.A 5-co2 band with a double bond is certainly calledcyclopentene.Hydrocarbons that consist of one or even more triple an actual are usually calledalkynes,and can be the name ending can be '-yne.' A 2-carbon alkyneis thus namedethyne.(However, the substance is often referred to by its common name, which isacetylene.)Substances that include dual or triple a genuine are stated to end up being'unsaturated'-because they are not'saturated' with hydrogén atoms. Unsaturated substances arereactive materials that easily add hydrogen when heated over acatalystsuch as nickel. The reverse reaction also occurs. Heating ethane withsteam will be an important commercial procedure for producing ethene (or ethylene).This is usually an important commercial process known as 'steamcracking.' When a 6-co2 ring consists of 2 double an actual, it is calledcyclohexadiene,but when it provides 3 dual bonds, it is certainly not called cyclohexatriene; this isbecause a 6-carbon ring with three dual bonds requires on a specific type ofstability.
The dual bonds become totally conjugated and nó longerbehave as dual bonds. The ring, identified as a 'benzene ring,'is stated to end up being aromatic.The removal of a hydrogén atom from á hydrocarbon molecule results in an alkylgroup that readily connects to a useful group, or types a department on ahydrocarbon chain. The organizations are named after the correspondinghydrocarbons. For example, CH3- is usually namedmethyI;CH3CH2-,ethyI;CH2= CH-,ethenyI;CH3CH2CH2-,propyl;and therefore on. A benzene band from which á hydrogen atom provides been taken out isoften referred to as aphenyI.The branched substances demonstrated here would end up being given names as follows. In theory there is no limit to the size of hydrocarbon stores.
Verylarge hydrocarbon substances (polymers) have been produced filled with as manyas 100,000 carbon atoms. Nevertheless, such molecules are difficult to create and verydifficult to melt and to form into helpful items.Hydrocarbons are usually obtained mainly from fossil fueIs-especiallypetroleum and natural gas. Organic gas can be a blend that will be largelymethane combined with differing quantities of ethane and additional lighting hydrocarbons,while oil is definitely a complicated combination of several various hydrocarbons. Coal,the additional fossil fuel, is definitely a very much more difficult materials from which manykinds of natural substances, some of thém hydrocarbons, can be obtained.Practical GroupsAlkane elements are rather unreactive (except for being pretty flammable),but alkenes react with numerous other chemicals. When a fall of bromine isaddéd to an aIkene, for illustration, the serious orange colour of the bromineimmediately goes away as the bromine provides across the double bond to forma dibromo type. The double bond will be called a 'functionalgroup' because its existence in a molecule leads to reactivity at thatparticular site.
There are usually a dozen or therefore functional groupings that appearfrequently in natural compounds. Some of the nearly all common ones are listedin Desk 2. The exact same molecule may consist of several useful groups.Aspirin, for instance, is definitely both acarboxylic ácidand anester, and choIesterol is an alkene simply because properly as an alcohol.IsomerismIsomers are elements with the exact same molecular formuIa but differentstructures. Thére is usually just one construction for methane, ethane, or gas;but butane, G4H10, can possess either of two different framework. In a conjugated system, there are alternating dual and one bonds,allowing electrons to flow back again and on. Substances that contain suchconjugated systems are said to end up being stable by'resonance.' ln the benzene band every additional bond is definitely adouble bond, all the way around the band.
This outcomes in a specific kindof stabilization called 'aromaticity,' in which theelectrons are usually delocalized and free to vacation all around the ring.Certain ring compounds, like benzene, that include like a conjugatedsystem of dual and individual bonds are referred to as'aromatic.' Pentane has 3 isomers: pentane (orn-pentane), methylbutane (oriso-pentane), and dimethyl lp (orneopentane). Hexane has 5 isomers:hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and2,3-dimethylbutane. Heptane provides 9 different isomers, octane has 18, nonanehas 35, and decane has 75. An increase in the amount of co2 atomsgreatly improves the opportunities for isomerism. There are more than4,000 isomers of C15H32and even more than 366,000 isomers of C20H42. The formula Chemical30H62has even more than 4 billion.
Of program, most of them have got never long been isolatedas real substances (but could be, if there were any stage in performing it).For molecules various other than hydrocarbons, nevertheless other kinds of isomers arepossible. The easy formula G2H6O can signify ethyl alcoholic beverages or dimethyl ether; and M3H6O could remain for an alcoholic beverages, an ether, anaIdehyde, or aketone(amóng various other points). The bigger a molecule is definitely, and the higher thevariety of atoms and functional groupings it includes, the even more several itsisomers.There is definitely nevertheless another kind of isomerism that stems from the life of'right-' and 'left-handed' elements.
It issometimes referred to asopticalisomerism because the molecules that create up a set of these isomersusually differ only in the method they rotate plane polarized lighting.NomenclatureThere are so several large numbers of organic compounds that just selecting namesfor them all will be a main problem. It has been not really until the late nineteenthcentury that chemists created a reasonable program for naming organiccompounds. Compounds had frequently been named based to their sources.
The1-co2 carboxylic acidity, for example, was first obtained from ants, andso it has been calledformicacid, from the Latin term for ants (formicae). The 2-carbon acid was acquired from vinegar (acetumin Latin), and was calledaceticacid.To bring some order to the naming procedure an worldwide meeting washeld in 1892 at Geneva, Switzerland.
The group later grew to become recognized as theInternational Marriage of Pure and Applied Chemistry (IUPAC). Its objectivewas to establish a naming procedure that would supply each substance with aunique and systematic title. An initial set of rules was followed at thatfirst conference in Geneva, and IUPAC provides continuing that work. Its systematicnaming rules are utilized by natural chemists all over the entire world. The brands ofthe alkanes type the time frame for the program, with practical groups usuallybeing indicated with appropriate suffixes.
Some illustrations are provided inTable 2.Organic ReactionsOrganic biochemistry is worried with the numerous substances of co2, theirnames, their isomérs, and their attributes, but it will be mainly concernedwith their reactions. Natural chemists have got developed a massive variety ofchemical responses that can convert one organic substance to another. Somereactions include add-on of one moIecule to another; somé involvedecomposition of molecules; some involve replacement of one atóm or groupby anothér; and some even involve the rearrangement of substances, withsome atoms relocating into new placements. Some reactions require power in theform of warmth or rays; and some require a exclusive type of switch orsome type. Table 2.NAMING Natural COMPOUNDSFunctional GroupType CompoundExampIeIUPAC NameCommon NameC=CdoubIe bondalkeneH2G=CH2etheneethyleneC≡Ctriple bondalkyneHC≡CHethyneacetylene-OHhydroxylalcoholCH3OHmethanolmethyl alcohol-O-oxyetherH3COCH3methoxymethanemethyl ethercarbonylaldehydeH2M=0methanalformaldehydecarbonylketoneCH3COCH3propanoneacetonecarboxylcarboxylic acidHCOOHmethanoic acidformic acidcarboxylesterHCOOCH2CH3ethyl methanoateethyl formate-NH2aminoamineCH3NH2aminomethanemethylamine-CNcyanonitrileCH3CNethanenitrileacetonitrile-XhalogenhaloalkaneCH3Clchloromethanemethyl chlorideof solvent. Of course, not all organic reactions are usually highly prosperous.One response might be a very easy one providing basically 100 percent ofthe desired item; but another might be a complicated multistep processyielding much less than 5 pct overall of the needed product.Organic reactions can often give remarkable handle as to what productsshould end up being formed.
Including drinking water to propene for instance, creates 2-propanolin the existence of acid solution, but it yields 1-propanol if treated very first with M2H6and then L2O2in the existence of base.Future Resources of Natural ChemicalsFossil fuels have been our main natural source for numerous organicchemicals for more than a hundred years, but our fossil gasoline assets arefinite, and they are being rapidly exhausted (especially essential oil and fuel). Whatwill be our sources of natural materials in the future? Since fossil fuelsare nonrenewable assets, it is certainly considered that the twénty-first centurywill notice a shift toward higher reliance on renewable raw materials. Thelargest U.T.
Chemical company offers a goal of becoming 25 pct centered onrenewable assets by 2010. It will be already generating 1,3-propanediol fromcornstarch using a gene-taiIoredE.